Research
Technology development: protein engineering tools for mammalian cell biology and engineering
We investigate strategies for monitoring specific protein conformational species in the complex environment of human cells and controlling the cellular level of virtually any protein with exquisite specificity and selectivity….READ MORE
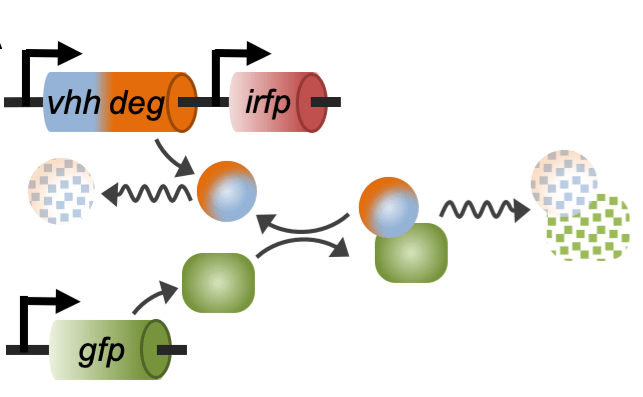
Engineering synthetic regulatory systems to reprogram mammalian cells
We develop innovative synthetic biology approaches to reprogram mammalian cells for the development of diagnostics and therapeutics. Mathematical modeling and experimental tests are integrated to optimize the design of orthogonal synthetic circuits for monitoring and manipulating pathways that maintain protein homeostasis. To this end, we leverage our extensive background in protein engineering to build much needed biological “parts” for mammalian synthetic biology. These novel tools are then combined through synthetic biology strategies to design innovative genetic networks that interface with innate cellular networks. The ultimate goals of this approach are to (i) generate sensitive reporters of fundamental biological pathways in a way that recapitulates the complexity of mammalian regulatory systems, and (ii) design strategies to manipulate these pathways through orthogonal genetic circuits that interface with the innate cellular machinery through highly sensitive feedback mechanisms.
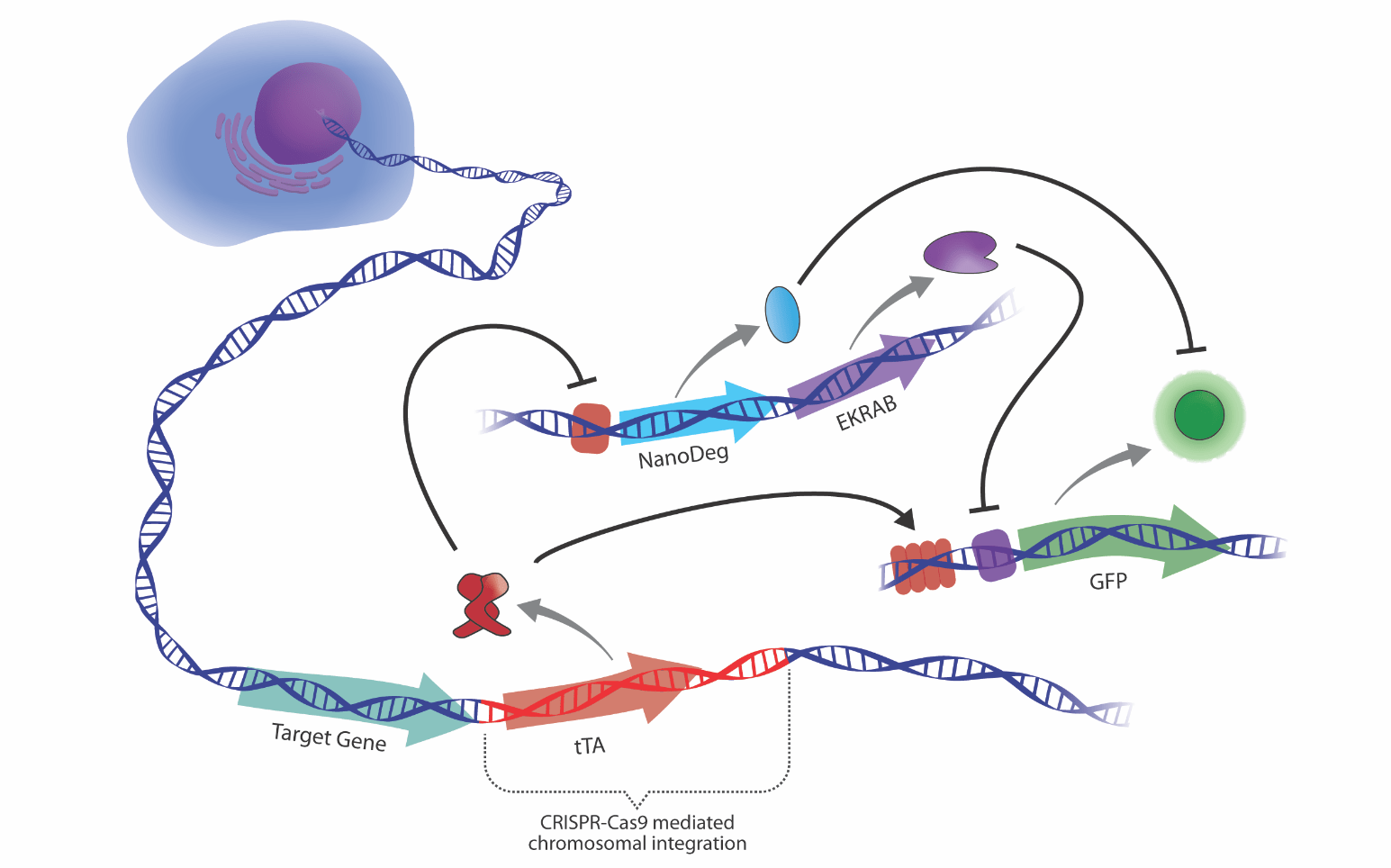
Design of cell-based therapies that sense and respond to transcriptional signatures
We explore innovative strategies for developing cellular devices that adjust the production of user-defined outputs in response to feedback signals generated by the device’s own gene activity, which is constantly and dynamically modulated to respond to environmental and intracellular stimuli. This approach is expected to create a paradigm shift in mammalian synthetic biology and to open the way to the design of smart cell therapies for self-regulated drug production (Nature Chem Biol. 2020 May;16(5):520-528).
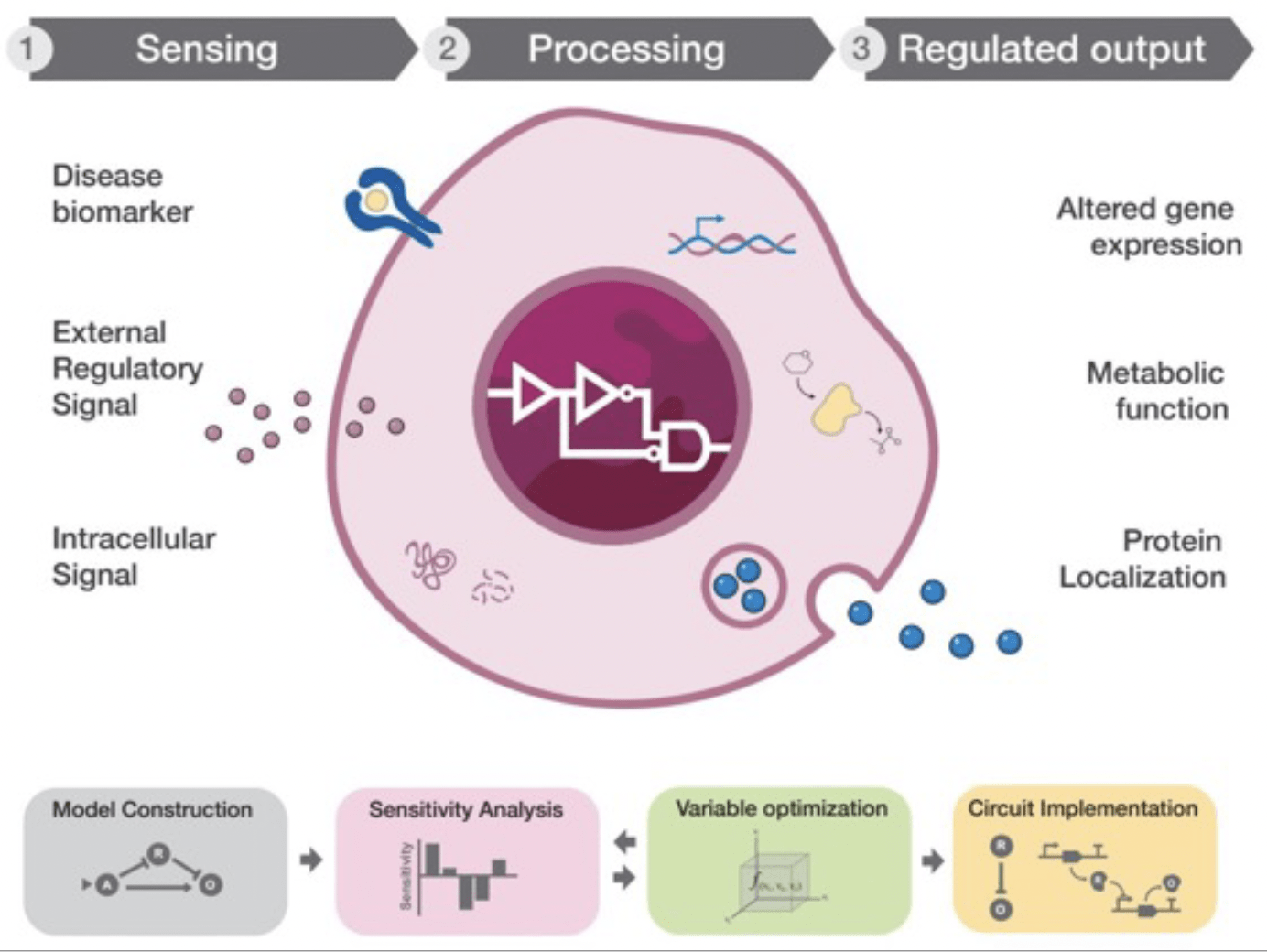
Next-generation, feedback-responsive cell factories for recombinant protein manufacturing
Recombinant proteins are the largest class of therapeutic drugs and are used for the treatment of a wide range of diseases. We develop synthetic biology strategies for monitoring and manipulating the cellular stress response to the overproduction of therapeutic proteins, with the ultimate goal of enhancing the innate cellular capacity to buffer proteotoxic stress. We aim to deploy this approach and decipher the regulatory mechanisms mediating the induction of the cellular stress response to engineer dynamic cell factories for improved biomanufacturing (Nature Chem Biol. 2020 May;16(5):520-528).
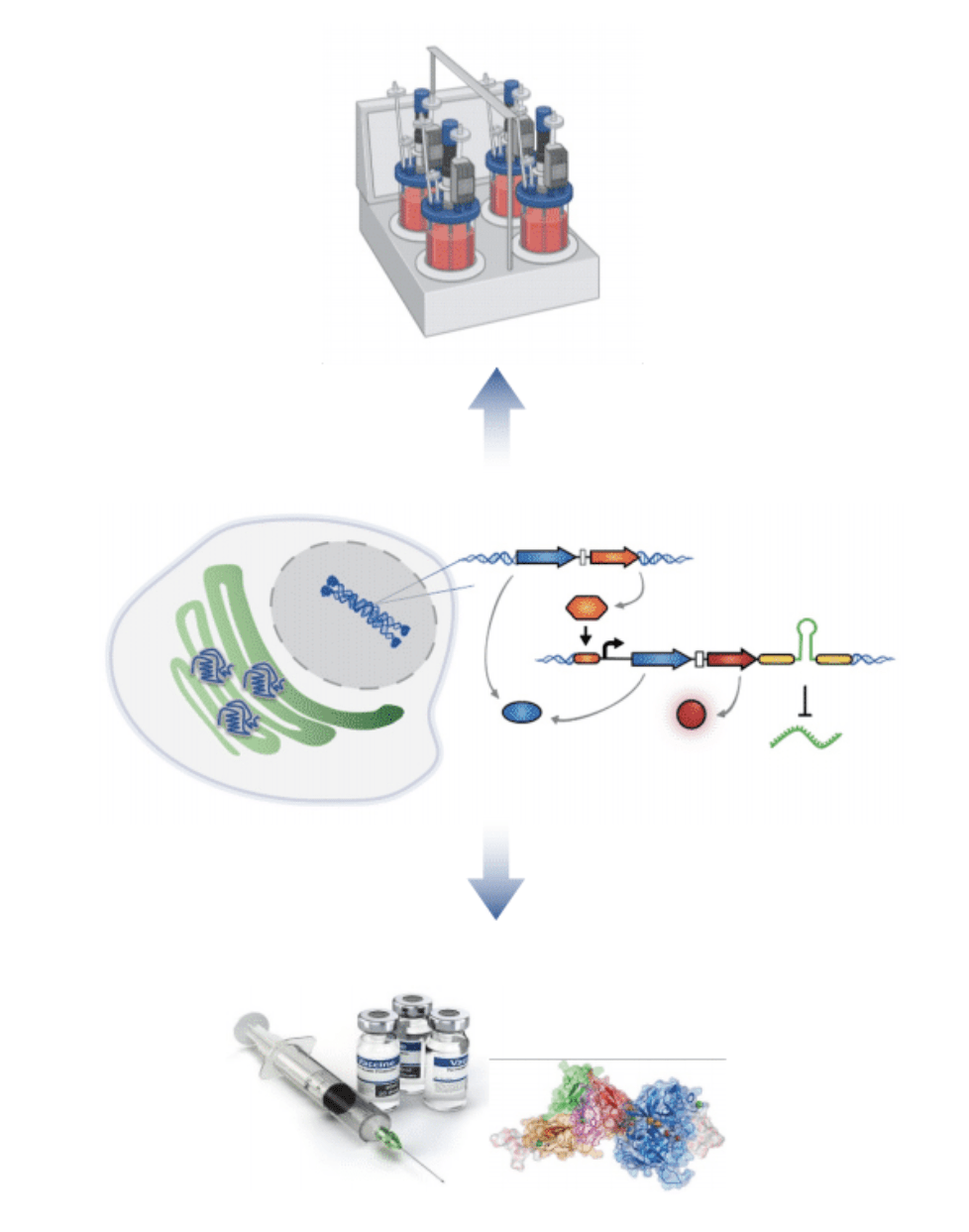
Sentinel cells: A plug-and-play RNA sensing technology platform for surveillance and response to emerging viral diseases
We develop living RNA sensors for applications in fundamental research and biomedicine. The potential of RNA detection in living cells for the development of RNA-based sensing devices remains largely unexplored due to existing gaps in fundamental knowledge of RNA biology and engineering. In collaboration with Dr. James Chappell (BioSciences, Rice U), we are building plug-and-play platforms for RNA sensing to build living sensors of pathogenic threats. We also integrate RNA sensors with synthetic gene circuits to create highly tunable sensor input-output response dynamics. We envision that this work will generate a universal platform that could be deployed for monitoring any RNA (viral and host RNAs), with a transformative impact on the design of sentinel cells that sense and respond to diverse physiological and pathological processes.
Understanding the interplay between engineered nanomaterials and the autophagy-lysosome system
Our understanding of pathways involved in cell quality control and clearance mechanisms and the experimental frameworks that we have developed to monitor the accumulation of toxic protein and lipid aggregates have provided the groundwork for a comprehensive research effort to investigate the impact of engineered nanomaterials on cells for applications in biomedicine and nanotechnology. We found that this cellular adaptive response to nanoparticle internalization may result in the clearance of toxic endogenous material that accumulates in association with disease development. By developing assays that link macroscopic phenomena (e.g., toxicity) with molecular mechanisms, we study the effectiveness and safety of nanomaterials. In addition to investigating nanoparticle-based therapeutic and diagnostic modalities, we are also exploring the potential health and safety risks of engineered nanomaterials commonly used in commercial products and/or released to the environment (ACS Nano. 2014 Oct 28;8(10):10328-42. Virology. 2017 Oct;510:1-8. Acta Biomaterialia. 2018 Oct 1;79:354-363. Bioconjug Chem. 2019 Jul 17;30(7):1986-1997).
Biological and chemical strategies to study and treat diseases of proteostasis deficiency
We demonstrated the use of chemical strategies to modulate the proteostasis network and restore the function of proteins whose deficiency is associated with the development of a range of human diseases. To elucidate the molecular mechanisms that mediate protein processing in cells, we have developed a series of strategies to modulate the proteostasis network in different model systems ranging from loss of function such as lysosomal storage disorders (J Biol Chem 2011 Dec 16;286(50):43454-64. ACS Chem Biol. 2013. 8 (7), 1460-1468. Hum Mol Genet 2013. May 15;22(10):1994-2009. Nat Cell Biol, 2018 Dec;20(12):1370-1377. doi: 10.1038/s41556-018-0228-7) to gain of toxic function, such as Parkinson’s disease (PLoS ONE. 2015 Mar 19;10(3):e0120819. ACS Chem Biol. 2013. 8 (7), 1460-1468).
Funding

